Atlanta Pediatric Research | NIH Requirements for Human Subject Research | Clinical Research Resources | Research Resources | Research | Emory + Children's + GT | Atlanta Pediatric Research Alliance
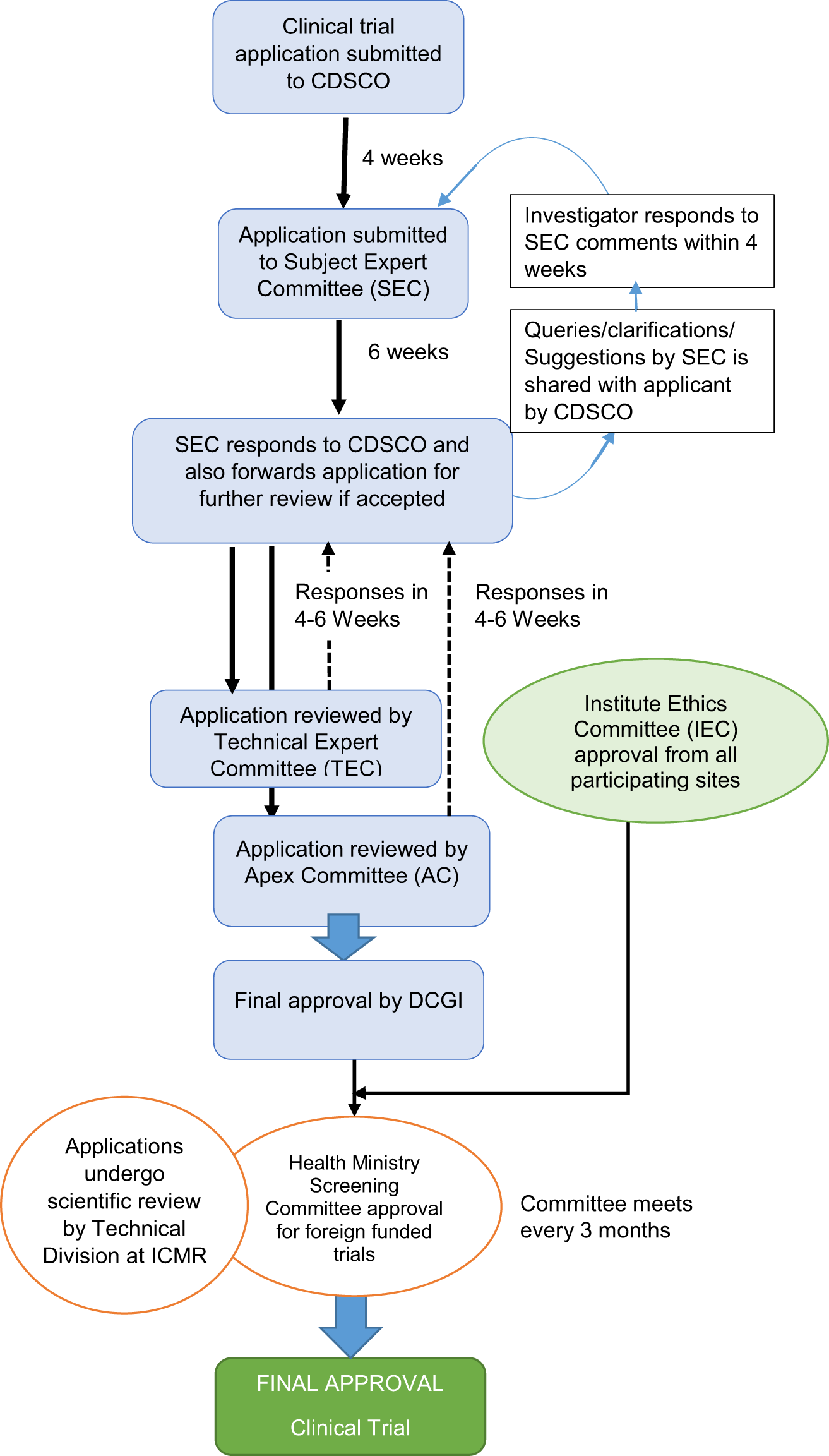
Issues, challenges, and the way forward in conducting clinical trials among neonates: investigators' perspective | Journal of Perinatology